Donor Pigs for Xenotransplantation
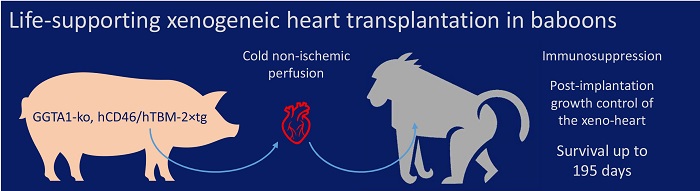
Transplantation is the only long-term intervention available for patients with terminal heart failure. However, the number of donated human organs falls far short of the need, a situation that threatens the life of many potential recipients. Alternative techniques such as xenotransplantation are therefore urgently needed. Hearts from genetically multi-modified pigs (GGTA1-ko, hCD46/hTBM 2xtg) functioned for up to 195 days after orthotopic transplantation (heart replacement) in baboons (Längin et al., Nature 2018). Consistent long-term success in life-supporting orthotopic porcine heart transplantation in the most relevant preclinical model is regarded as a milestone on the way to clinical cardiac xenotransplantation.
The number of donated human organs and tissues falls far short of the need, a situation that threatens the life of many potential recipients. Alternative techniques such as xenotransplantation are therefore urgently needed. The pig is the preferred donor species for a number of reasons, including size, anatomical and physiological similarities with humans, and efficient techniques for genetic engineering/gene editing. Technical advances in the generation of genetically multi-modified pigs and new developments in the field of immunosuppression have supported significant progress in many areas of xenotransplantation, including pancreatic islets, neuronal cells, and corneas, but also vascularized organs, especially kidney and heart.
Within the DFG-funded Transregional Collaborative Research Center 127 “Biology of xenogeneic cell, tissue and organ transplantation – from bench to bedside” (http://www.klinikum.uni-muenchen.de/SFB-TRR-127/de/) we are generating genetically multi-modified pigs to overcome rejection mechanisms and physiological incompatibilities of pig-to-primate xenografts.
Highlights
- Spatial distribution of beta cells in total cleared porcine pancreas revealed (Zhao et al., Cell 2020)
- Consistent success in life-supporting cardiac xenotransplantation (Längin et al., Nature 2018)
- INS-eGFP transgenic pigs for studying beta-cell maturation and expansion (Kemter et al., Diabetologia 2017)
- Immuneprotection of xenotransplanted porcine islets by local expression of LEA29Y (Klymiuk et al., Diabetes 2012; Wolf-van Bürck et al., Sci Rep 2017)
Publications
2023
Fischer N, Gulich B, Keßler B, Längin M, Fishman JA, Wolf E, Boller K, Tönjes RR, Godehardt AW. PCR and peptide based PCMV detection in pig - development and application of a combined testing procedure differentiating newly from latent infected pigs. Xenotransplantation. 2023 Apr 30:e12803. doi: 10.1111/xen.12803. Epub ahead of print. PMID: 37120823
Reichart B, Cooper DKC, Längin M, Tönjes RR, Pierson RN, Wolf E. CARDIAC XENOTRANSPLANTATION - FROM CONCEPT TO CLINIC. Cardiovasc Res. 2022 Dec 3:cvac180. PMID: 36461918
von Bibra C, Shibamiya A, Bähr A, Geertz B, Köhne M, Stuedemann T, Starbatty J, Horneffer-van der Sluis V, Klostermeier UC, Hornaschewitz N, Li X, Wolf E, Klymiuk N, Krane M, Kupatt C, Hiebl B, Eschenhagen T, Weinberger F. Immature human engineered heart tissues engraft in a guinea pig chronic injury model. Dis Model Mech. 2023 May 1;16(5):dmm049834. doi: 10.1242/dmm.049834. Epub 2023 Jun 5. PMID: 37272385; PMCID: PMC10259837
Wolf E, Reichart B, Moretti A, Laugwitz KL. Designer pigs for xenogeneic heart transplantation and beyond. Dis Model Mech. 2023 May 1;16(5):dmm050177. doi: 10.1242/dmm.050177. Epub 2023 May 30. PMID: 37249503; PMCID: PMC10245136
2022
Bender M, Längin M, Reichart B, Mokelke M, Radan J, Neumann E, Michel S, Ellgass R, Cowan PJ, Wolf E, Abicht JM, Brenner P. Overcoming perioperative inflammation as a hurdle for successful preclinical orthotopic cardiac xenogeneic transplantations - particular in regard of the mandatory use of heart-lung machines. Xenotransplantation. 2022 May;29(3):e12749. PMID: 35616211
Bender M, Längin M, Reichart B, Mokelke M, Radan J, Neumann E, Michel S, Ellgass R, Cowan PJ, Wolf E, Abicht JM, Brenner P. Clinical cardiac xenotransplantation first in the clinical arena. Xenotransplantation. 2022 Jan;29(1):e12734. PMID: 35165939
Duin S, Bhandarkar S, Lehmann S, Kemter E, Wolf E, Gelinsky M, Ludwig B, Lode A. Viability and Functionality of Neonatal Porcine Islet-like Cell Clusters Bioprinted in Alginate-Based Bioinks. Biomedicines. 2022 Jun 15;10(6):1420. PMID: 35740440
Fehér A, Schnúr A, Muenthaisong S, Bellák T, Ayaydin F, Várady G, Kemter E, Wolf E, Dinnyés A. Establishment and characterization of a novel human induced pluripotent stem cell line stably expressing the iRFP720 reporter. Sci Rep. 2022 Jun 14;12(1):9874. PMID: 35701501
Kemter E, Citro A, Wolf-van Buerck L, Qiu Y, Böttcher A, Policardi M, Pellegrini S, Valla L, Alunni-Fabbroni M, Kobolák J, Kessler B, Kurome M, Zakhartchenko V, Dinnyes A, Cyran CC, Lickert H, Piemonti L, Seissler J, Wolf E. Transgenic pigs expressing near infrared fluorescent protein-A novel tool for noninvasive imaging of islet xenotransplants. Xenotransplantation. 2022 Jan;29(1):e12719. PMID: 34935207
Konstantinov IE, Cooper DKC, Adachi I, Bacha E, Bleiweis MS, Chinnock R, Cleveland D, Cowan PJ, Fynn-Thompson F, Morales DLS, Mohiuddin MM, Reichart B, Rothblatt M, Roy N, Turek JW, Urschel S, West L, Wolf E. Consensus statement on heart xenotransplantation in children: Toward clinical translation. J Thorac Cardiovasc Surg. 2022 Sep 6:S0022-5223(22)00926-6. PMID: 36184321
2021
Hawthorne WJ, Cowan PJ, Buhler L, Wolf E. International standards and guidelines for xenotransplantation. Nat Biotechnol. 2021 Dec;39(12):1501-1502. PMID: 34857929
Hinrichs A, Riedel EO, Klymiuk N, Blutke A, Kemter E, Längin M, Dahlhoff M, Keßler B, Kurome M, Zakhartchenko V, Jemiller EM, Ayares D, Bidlingmaier M, Flenkenthaler F, Hrabĕ de Angelis M, Arnold GJ, Reichart B, Fröhlich T, Wolf E. Growth hormone receptor knockout to reduce the size of donor pigs for preclinical xenotransplantation studies. Xenotransplantation. 2021 Mar;28(2):e12664. PMID: 33241624
Reichart B, Längin M, Denner J, Schwinzer R, Cowan PJ, Wolf E. Pathways to Clinical Cardiac Xenotransplantation. Transplantation. 2021 Sep 1;105(9):1930-1943. PMID: 33350675
Zhang Y, Lei Y, Honarpisheh M, Kemter E, Wolf E, Seissler J. Butyrate and Class I Histone Deacetylase Inhibitors Promote Differentiation of Neonatal Porcine Islet Cells into Beta Cells. Cells. 2021 Nov 19;10(11):3249. PMID: 34831471
2020
Denner J, Längin M, Reichart B, Krüger L, Fiebig U, Mokelke M, Radan J, Mayr T, Milusev A, Luther F, Sorvillo N, Rieben R, Brenner P, Walz C, Wolf E, Roshani B, Stahl-Hennig C, Abicht JM. Impact of porcine cytomegalovirus on long-term orthotopic cardiac xenotransplant survival. Sci Rep 2020; 10:17531
Fischer K, Rieblinger B, Hein R, Sfriso R, Zuber J, Fischer A, Klinger B, Liang W, Flisikowski K, Kurome M, Zakhartchenko V, Kessler B, Wolf E, Rieben R, Schwinzer R, Kind A, Schnieke A. Viable pigs after simultaneous inactivation of porcine MHC class I and three xenoreactive antigen genes GGTA1, CMAH and B4GALNT2. Xenotransplantation 2020; 27:e12560
Kemter E, Schnieke A, Fischer K, Cowan PJ, Wolf E. Xeno-organ donor pigs with multiple genetic modifications - the more the better? Curr Opin Genet Dev 2020; 64:60-65
Längin M, Reichart B, Steen S, Sjöberg T, Paskevicius A, Liao Q, Qin G, Mokelke M, Mayr T, Radan J, Issl L, Buttgereit I, Ying J, Fresch AK, Panelli A, Egerer S, Bähr A, Kessler B, Milusev A, Sfriso R, Rieben R, Ayares D, Murray PJ, Ellgass R, Walz C, Klymiuk N, Wolf E, Abicht JM, Brenner P. Cold non-ischemic heart preservation with continuous perfusion prevents early graft failure in orthotopic pig-to-baboon xenotransplantation. Xenotransplantation 2020:e12636
Ramackers W, Rataj D, Werwitzke S, Bergmann S, Winkler M, Wuensch A, Baehr A, Wolf E, Klymiuk N, Ayares D, Tiede A. Expression of human thrombomodulin on porcine endothelial cells can reduce platelet aggregation but did not reduce activation of complement or endothelium - an experimental study. Transpl Int 2020; 33:437-449
Reichart B, Längin M, Denner J, Schwinzer R, Cowan PJ, Wolf E. Pathways to Clinical Cardiac Xenotransplantation. Transplantation 2020a; Dec 21; Online Ahead of Print. doi: 10.1097/TP.0000000000003588.
Reichart B, Längin M, Radan J, Mokelke M, Buttgereit I, Ying J, Fresch AK, Mayr T, Issl L, Buchholz S, Michel S, Ellgass R, Mihalj M, Egerer S, Baehr A, Kessler B, Kemter E, Kurome M, Zakhartchenko V, Steen S, Sjöberg T, Paskevicius A, Krüger L, Fiebig U, Denner J, Godehardt AW, Tönjes RR, Milusev A, Rieben R, Sfriso R, Walz C, Kirchner T, Ayares D, Lampe K, Schönmann U, Hagl C, Wolf E, Klymiuk N, Abicht JM, Brenner P. Pig-to-non-human primate heart transplantation: The final step toward clinical xenotransplantation? J Heart Lung Transplant 2020b; 39:751-757
Wuensch A, Kameritsch P, Sfriso R, Jemiller EM, Baehr A, Kurome M, Kessler B, Kemter E, Kupatt C, Reichart B, Rieben R, Wolf E, Klymiuk N. Genetically encoded Ca(2+) -sensor reveals details of porcine endothelial cell activation upon contact with human serum. Xenotransplantation 2020:e12585
Zhao S, Todorov MI, Cai R, Maskari RA, Steinke H, Kemter E, Mai H, Rong Z, Warmer M, Stanic K, Schoppe O, Paetzold JC, Gesierich B, Wong MN, Huber TB, Duering M, Bruns OT, Menze B, Lipfert J, Puelles VG, Wolf E, Bechmann I, Erturk A. Cellular and Molecular Probing of Intact Human Organs. Cell 2020; 180:796-812.e719
2019
Hawthorne WJ, Cowan PJ, Buhler LH, Yi S, Bottino R, Pierson RN, 3rd, Ahn C, Azimzadeh A, Cozzi E, Gianello P, Lakey JRT, Luo M, Miyagawa S, Mohiuddin MM, Park CG, Schuurman HJ, Scobie L, Sykes M, Tector J, Tonjes RR, Wolf E, Nunez JR, Wang W. Third WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials, Changsha, Hunan, China December 12-14, 2018: "The 2018 Changsha Communique" The 10-Year Anniversary of The International Consultation on Xenotransplantation. Xenotransplantation 2019; 26:e12513
Krueger L, Laengin M, Reichart B, Fiebig U, Kristiansen Y, Prinz C, Kessler B, Egerer S, Wolf E, Abicht JM, Denner J. Transmission of Porcine Circovirus 3 (PCV3) by Xenotransplantation of Pig Hearts into Baboons. Viruses 2019; 11
Wolf E, Kemter E, Klymiuk N, Reichart B. Genetically modified pigs as donors of cells, tissues, and organs for xenotransplantation. Anim Front 2019; 9:13-20
2018
Abicht JM, Sfriso R, Reichart B, Laengin M, Gahle K, Puga Yung GL, Seebach JD, Rieben R, Ayares D, Wolf E, Klymiuk N, Baehr A, Kind A, Mayr T, Bauer A. Multiple genetically modified GTKO/hCD46/HLA-E/hbeta2-mg porcine hearts are protected from complement activation and natural killer cell infiltration during ex vivo perfusion with human blood. Xenotransplantation 2018; 25:e12390
Egerer S, Fiebig U, Kessler B, Zakhartchenko V, Kurome M, Reichart B, Kupatt C, Klymiuk N, Wolf E, Denner J, Baehr A. Early weaning completely eliminates porcine cytomegalovirus from a newly established pig donor facility for xenotransplantation. Xenotransplantation 2018; 25:e12449
Fiebig U, Abicht JM, Mayr T, Laengin M, Baehr A, Guethoff S, Falkenau A, Wolf E, Reichart B, Shibahara T, Denner J. Distribution of Porcine Cytomegalovirus in Infected Donor Pigs and in Baboon Recipients of Pig Heart Transplantation. Viruses 2018a; 10
Fiebig U, Fischer K, Baehr A, Runge C, Schnieke A, Wolf E, Denner J. Porcine endogenous retroviruses: Quantification of the copy number in cell lines, pig breeds, and organs. Xenotransplantation 2018b; 25:e12445
Kemter E, Denner J, Wolf E. Will Genetic Engineering Carry Xenotransplantation of Pig Islets to the Clinic? Current diabetes reports 2018; 18:103
Kemter E, Wolf E. Recent progress in porcine islet isolation, culture and engraftment strategies for xenotransplantation. Current opinion in organ transplantation 2018; 23:633-641
Längin M, Mayr T, Reichart B, Michel S, Buchholz S, Guethoff S, Dashkevich A, Baehr A, Egerer S, Bauer A, Mihalj M, Panelli A, Issl L, Ying J, Fresch AK, Buttgereit I, Mokelke M, Radan J, Werner F, Lutzmann I, Steen S, Sjoberg T, Paskevicius A, Qiuming L, Sfriso R, Rieben R, Dahlhoff M, Kessler B, Kemter E, Kurome M, Zakhartchenko V, Klett K, Hinkel R, Kupatt C, Falkenau A, Reu S, Ellgass R, Herzog R, Binder U, Wich G, Skerra A, Ayares D, Kind A, Schoenmann U, Kaup FJ, Hagl C, Wolf E, Klymiuk N, Brenner P, Abicht JM. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018; 564:430-433
Puga Yung G, Bongoni AK, Pradier A, Madelon N, Papaserafeim M, Sfriso R, Ayares DL, Wolf E, Klymiuk N, Baehr A, Constantinescu MA, Voegelin E, Kiermeir D, Jenni H, Rieben R, Seebach JD. Release of pig leukocytes and reduced human NK cell recruitment during ex vivo perfusion of HLA-E/human CD46 double-transgenic pig limbs with human blood. Xenotransplantation 2018; 25
Rieblinger B, Fischer K, Kind A, Saller BS, Baars W, Schuster M, Wolf-van Buerck L, Schaeffler A, Flisikowska T, Kurome M, Zakhartchenko V, Kessler B, Flisikowski K, Wolf E, Seissler J, Schwinzer R, Schnieke A. Strong xenoprotective function by single-copy transgenes placed sequentially at a permissive locus. Xenotransplantation 2018; 25:e12382
2017
Beshr G, Sikandar A, Jemiller EM, Klymiuk N, Hauck D, Wagner S, Wolf E, Koehnke J, Titz A. Photorhabdus luminescens lectin A (PllA): A new probe for detecting alpha-galactoside-terminating glycoconjugates. The Journal of biological chemistry 2017; 292:19935-19951
Bongoni AK, Salvaris E, Nordling S, Klymiuk N, Wolf E, Ayares DL, Rieben R, Magnusson PU, Cowan PJ. Surface modification of pig endothelial cells with a branched heparin conjugate improves their compatibility with human blood. Scientific reports 2017; 7:4450
Cohrs CM, Chen C, Jahn SR, Stertmann J, Chmelova H, Weitz J, Baehr A, Klymiuk N, Steffen A, Ludwig B, Kamvissi V, Wolf E, Bornstein SR, Solimena M, Speier S. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology 2017; 158:1373-1385
Kemter E, Cohrs CM, Schaefer M, Schuster M, Steinmeyer K, Wolf-van Buerck L, Wolf A, Wuensch A, Kurome M, Kessler B, Zakhartchenko V, Loehn M, Ivashchenko Y, Seissler J, Schulte AM, Speier S, Wolf E. INS-eGFP transgenic pigs: a novel reporter system for studying maturation, growth and vascularisation of neonatal islet-like cell clusters. Diabetologia 2017a; 60:1152-1156
Puga Yung G, Bongoni AK, Pradier A, Madelon N, Papaserafeim M, Sfriso R, Ayares DL, Wolf E, Klymiuk N, Baehr A, Constantinescu MA, Voegelin E, Kiermeir D, Jenni H, Rieben R, Seebach JD. Release of pig leukocytes and reduced human NK cell recruitment during ex vivo perfusion of HLA-E/human CD46 double-transgenic pig limbs with human blood. Xenotransplantation 2017
Sfriso R, Bongoni A, Banz Y, Klymiuk N, Wolf E, Rieben R. Assessment of the Anticoagulant and Anti-inflammatory Properties of Endothelial Cells Using 3D Cell Culture and Non-anticoagulated Whole Blood. Journal of visualized experiments : JoVE 2017
Wolf-van Buerck L, Schuster M, Oduncu FS, Baehr A, Mayr T, Guethoff S, Abicht J, Reichart B, Klymiuk N, Wolf E, Seissler J. LEA29Y expression in transgenic neonatal porcine islet-like cluster promotes long-lasting xenograft survival in humanized mice without immunosuppressive therapy. Scientific reports 2017; 7:3572
2016
Baehr A, Kaser T, Kemter E, Gerner W, Kurome M, Baars W, Herbach N, Witter K, Wuensch A, Talker SC, Kessler B, Nagashima H, Saalmuller A, Schwinzer R, Wolf E, Klymiuk N. Ubiquitous LEA29Y expression blocks T cell co-stimulation but permits sexual reproduction in genetically modified pigs. PloS one 2016; 11:e0155676
Bongoni AK, Klymiuk N, Wolf E, Ayares D, Rieben R, Cowan PJ. Transgenic expression of human thrombomodulin inhibits HMGB1-induced porcine aortic endothelial cell activation. Transplantation 2016; 100:1871-1879
Cooper DK, Matsumoto S, Abalovich A, Itoh T, Mourad NI, Gianello PR, Wolf E, Cozzi E. Progress in clinical encapsulated islet xenotransplantation. Transplantation 2016; 100:2301-2308
Cowan PJ, Ayares D, Wolf E, Cooper DK. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--Chapter 2b: genetically modified source pigs. Xenotransplantation 2016; 23:32-37
Fischer K, Kraner-Scheiber S, Petersen B, Rieblinger B, Buermann A, Flisikowska T, Flisikowski K, Christan S, Edlinger M, Baars W, Kurome M, Zakhartchenko V, Kessler B, Plotzki E, Szczerbal I, Switonski M, Denner J, Wolf E, Schwinzer R, Niemann H, Kind A, Schnieke A. Efficient production of multi-modified pigs for xenotransplantation by 'combineering', gene stacking and gene editing. Scientific reports 2016; 6:29081
Mohiuddin MM, Singh AK, Corcoran PC, Thomas ML, 3rd, Clark T, Lewis BG, Hoyt RF, Eckhaus M, Pierson RN, 3rd, Belli AJ, Wolf E, Klymiuk N, Phelps C, Reimann KA, Ayares D, Horvath KA. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nature communications 2016; 7:11138
Rataj D, Werwitzke S, Haarmeijer B, Winkler M, Ramackers W, Petersen B, Niemann H, Wuensch A, Baehr A, Klymiuk N, Wolf E, Abicht JM, Ayares D, Tiede A. Inhibition of complement component C5 prevents clotting in an ex vivo model of xenogeneic activation of coagulation. Xenotransplantation 2016; 23:117-127
2015
Abicht JM, Mayr T, Reichart B, Buchholz S, Werner F, Lutzmann I, Schmoeckel M, Bauer A, Thormann M, Langenmayer M, Herbach N, Pohla H, Herzog R, McGregor CG, Ayares D, Wolf E, Klymiuk N, Baehr A, Kind A, Hagl C, Ganswindt U, Belka C, Guethoff S, Brenner P. Pre-clinical heterotopic intrathoracic heart xenotransplantation: a possibly useful clinical technique. Xenotransplantation 2015; 22:427-442
Bongoni AK, Kiermeir D, Schnider J, Jenni H, Garimella P, Bahr A, Klymiuk N, Wolf E, Ayares D, Voegelin E, Constantinescu MA, Seebach JD, Rieben R. Transgenic expression of human CD46 on porcine endothelium: effect on coagulation and fibrinolytic cascades during ex vivo human-to-pig limb xenoperfusions. Transplantation 2015; 99:2061-2069
Iwase H, Ekser B, Satyananda V, Bhama J, Hara H, Ezzelarab M, Klein E, Wagner R, Long C, Thacker J, Li J, Zhou H, Jiang M, Nagaraju S, Zhou H, Veroux M, Bajona P, Wijkstrom M, Wang Y, Phelps C, Klymiuk N, Wolf E, Ayares D, Cooper DK. Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation 2015; 22:211-220
Kemter E, Wolf E. Pigs pave a way to de novo formation of functional human kidneys. Proceedings of the National Academy of Sciences of the United States of America 2015; 112:12905-12906
Plotzki E, Wolf-van Buerck L, Knauf Y, Becker T, Maetz-Rensing K, Schuster M, Baehr A, Klymiuk N, Wolf E, Seissler J, Denner J. Virus safety of islet cell transplantation from transgenic pigs to marmosets. Virus research 2015; 204:95-102
Reichart B, Guethoff S, Brenner P, Poettinger T, Wolf E, Ludwig B, Kind A, Mayr T, Abicht JM. Xenotransplantation of cells, tissues, organs and the German Research Foundation Transregio Collaborative Research Centre 127. Advances in experimental medicine and biology 2015a; 865:143-155
Reichart B, Niemann H, Chavakis T, Denner J, Jaeckel E, Ludwig B, Marckmann G, Schnieke A, Schwinzer R, Seissler J, Toenjes RR, Klymiuk N, Wolf E, Bornstein SR. Xenotransplantation of porcine islet cells as a potential option for the treatment of type 1 diabetes in the future. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 2015b; 47:31-35
Wolf-van Buerck L, Schuster M, Baehr A, Mayr T, Guethoff S, Abicht J, Reichart B, Nam-Apostolopoulos YC, Klymiuk N, Wolf E, Seissler J. Engraftment and reversal of diabetes after intramuscular transplantation of neonatal porcine islet-like clusters. Xenotransplantation 2015; 22:443-450
Wolf E, Reichart B. Commentary on "Meta-analysis of the independent and cumulative effects of multiple genetic modifications on pig lung xenograft performance during ex vivo perfusion with human blood" (by Harris et al.): tailoring donor pigs for xenotransplantation-how to find the right combination of genetic modifications? Xenotransplantation 2015; 22:112-113
2014
Bongoni AK, Kiermeir D, Jenni H, Baehr A, Ayares D, Klymiuk N, Wolf E, Voegelin E, Constantinescu MA, Seebach JD, Rieben R. Complement dependent early immunological responses during ex vivo xenoperfusion of hCD46/HLA-E double transgenic pig forelimbs with human blood. Xenotransplantation 2014; 21:230-243
Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, 3rd, Lewis BG, Eckhaus M, Reimann KA, Klymiuk N, Wolf E, Ayares D, Horvath KA. One-year heterotopic cardiac xenograft survival in a pig to baboon model. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2014; 14:488-489
Wuensch A, Baehr A, Bongoni AK, Kemter E, Blutke A, Baars W, Haertle S, Zakhartchenko V, Kurome M, Kessler B, Faber C, Abicht JM, Reichart B, Wanke R, Schwinzer R, Nagashima H, Rieben R, Ayares D, Wolf E, Klymiuk N. Regulatory sequences of the porcine THBD gene facilitate endothelial-specific expression of bioactive human thrombomodulin in single- and multitransgenic pigs. Transplantation 2014; 97:138-147
2013
Bongoni AK, Kiermeir D, Jenni H, Wuensch A, Baehr A, Ayares D, Seebach JD, Wolf E, Klymiuk N, Constantinescu MA, Vogelin E, Rieben R. Activation of the lectin pathway of complement in pig-to-human xenotransplantation models. Transplantation 2013; 96:791-799
2012
Kemter E, Lieke T, Kessler B, Kurome M, Wuensch A, Summerfield A, Ayares D, Nagashima H, Baars W, Schwinzer R, Wolf E. Human TNF-related apoptosis-inducing ligand-expressing dendritic cells from transgenic pigs attenuate human xenogeneic T cell responses. Xenotransplantation 2012; 19:40-51
Klymiuk N, van Buerck L, Baehr A, Offers M, Kessler B, Wuensch A, Kurome M, Thormann M, Lochner K, Nagashima H, Herbach N, Wanke R, Seissler J, Wolf E. Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes 2012c; 61:1527-1532
2010
Aigner B, Klymiuk N, Wolf E. Transgenic pigs for xenotransplantation: selection of promoter sequences for reliable transgene expression. Current opinion in organ transplantation 2010a; 15:201-206
Klymiuk N, Aigner B, Brem G, Wolf E. Genetic modification of pigs as organ donors for xenotransplantation. Molecular reproduction and development 2010; 77:209-221
2009
Dieckhoff B, Kessler B, Jobst D, Kues W, Petersen B, Pfeifer A, Kurth R, Niemann H, Wolf E, Denner J. Distribution and expression of porcine endogenous retroviruses in multi-transgenic pigs generated for xenotransplantation. Xenotransplantation 2009; 16:64-73
Weiss EH, Lilienfeld BG, Mueller S, Mueller E, Herbach N, Kessler B, Wanke R, Schwinzer R, Seebach JD, Wolf E, Brem G. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation 2009; 87:35-43
2008
Klymiuk N, Wolf E, Aigner B. Concise classification of the genomic porcine endogenous retroviral gamma1 load to defined lineages. Virology 2008; 371:175-184
2005
Kemter E, Philipp U, Klose R, Kuiper H, Boelhauve M, Distl O, Wolf E, Leeb T. Molecular cloning, expression analysis and assignment of the porcine tumor necrosis factor superfamily member 10 gene (TNFSF10) to SSC13q34-->q36 by fluorescence in situ hybridization and radiation hybrid mapping. Cytogenetic and genome research 2005; 111:74-78
Klose R, Kemter E, Bedke T, Bittmann I, Kessler B, Endres R, Pfeffer K, Schwinzer R, Wolf E. Expression of biologically active human TRAIL in transgenic pigs. Transplantation 2005; 80:222-230

